인류의 건강과 생명공학 발전을 위해 봉사하는 기업 드림셀
We Serving the Health and Biotechnology of Humanity
We Serving the Health and Biotechnology of Humanity

제품코드 : CSB-EL3325HU
제품정보 : Human SARS-CoV-2 N IgG Antibody
| Description | CUSABIO's Human SARS-CoV-2 nucleoprotein (N) IgG Antibody ELISA Kit allows for in vitro qualitative detection of the IgG antibody-specific for SARS-CoV-2 N protein in human serum and plasma. This research-use only kit can be applied in the studies associated with COVID-19, infectious and severe pneumonia caused by SARS-CoV-2. It has been validated with high sensitivity, high specificity, and high precision. Nucleoprotein is the most abundant protein in SARS-CoV-2. It packs the positive-sense single-stranded viral RNA into a helical ribonucleocapsid (RNP), forming the viral nucleocapsid (N). Th...Read more | |||||||||||||||
| Alternative Names | N; Nucleoprotein; N; Nucleocapsid protein; NC; Protein N | |||||||||||||||
| Abbreviation | SARS-CoV-2 N Ab (IgG) | |||||||||||||||
| Uniprot No. | P0DTC9 | |||||||||||||||
| Species | Homo sapiens (Human) | |||||||||||||||
| Sample Types | serum, plasma | |||||||||||||||
| Assay Time | 1-5h | |||||||||||||||
| Sample Volume | 50-100ul | |||||||||||||||
| Detection Wavelength | 450 nm | |||||||||||||||
| Research Area | Infectious Diseases | |||||||||||||||
| Assay Principle | qualitative | |||||||||||||||
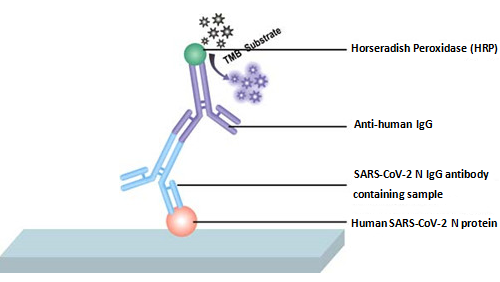 This assay mechanism is based on the qualitative enzyme immunoassay technique. The microtiter plate has been pre-coated with human SARS-CoV-2 nucleoprotein. Samples are pipetted into the wells with anti-human IgG conjugated HRP. Following a thorough wash, the TMB Substrate solution is added to the wells, eliciting a color change. The color develops in proportion to the amount of human SARS-CoV-2 N IgG antibody bound in the initial step. The addition of the stop solution to the wells terminates the color reaction. The color intensity can be measured at 450 nm via a microplate reader. The SARS-CoV-2 N IgG antibody titer in the samples is determined by referring to the negative control. It indicates the presence of SARS-CoV-2 N IgG antibody if the sample O.D. (optical density) is greater than or equal to the cutoff value (2.1×Average O.D. value of negative control). There is no SARS-CoV-2 N IgG antibody present in the sample if the O.D. is less than the cutoff value. | ||||||||||||||||
| Precision |
| |||||||||||||||
| Typical Data |
| |||||||||||||||
| Materials provided |
| |||||||||||||||
| Materials not provided |
| |||||||||||||||
| Troubleshooting and FAQs | ELISA kit FAQs | |||||||||||||||
| Storage | Store at 2-8°C. Please refer to protocol. | |||||||||||||||
| Lead Time | 3-5 working days | |||||||||||||||